Covid-19 self-test
COVID-19 antigenic or serological self-test. Detects if you are a carrier or have been a carrier of Covid-19.
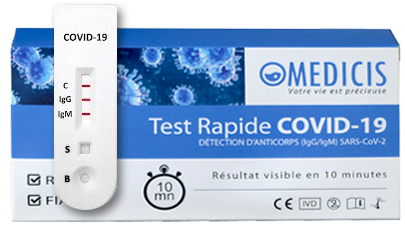
Covid-19 Serological Self-Test
This serological self-test determines if you are a carrier, or have had contact with the Coronavirus (up to 3 months ago). Fast and effective, the result is visible in 10 minutes.
Serological test approved by the National Reference Centre (CNR) of the Pasteur Institute, validated by the Ministry of Health. Self-test in compliance with CE standards. Discover the serological test.
Covid-19 Antigenic Autotest
This antigenic self-test determines whether you are a carrier of the Coronavirus. For your convenience, it allows several sampling options (oral and nasal). The result is visible in 20 minutes.
Test approved by the National Reference Centre (CNR) of the Pasteur Institute, validated by the Ministry of Health. Test conforms to CE standards. Discover the antigenic self-test.
They testify:
I am a manager in a transport company, and I have to check regularly if my employees are carrying the Covid-19. Indeed, the consequences would be disastrous if we were all contaminated, it would be the closure of my company for a few months! The Covid-19 test is really practical because in a few minutes you can get a first level of information and react as soon as the test is positive. It is a complement to PCR tests.
I was confined with my children during the crisis. My son had all the symptoms of Covid, including a very high fever. For my part, I had more moderate symptoms. I wanted to do a test and I was very surprised to see that the result was negative. This seems to indicate that my son did not get the COVID, but something else…
I wanted to get tested because several of my family members have tested positive for COVID-19. I wanted to know if I too was one of those people. The test was negative. I know now that I have to be careful and protect myself.
Frequently asked questions about self-tests :
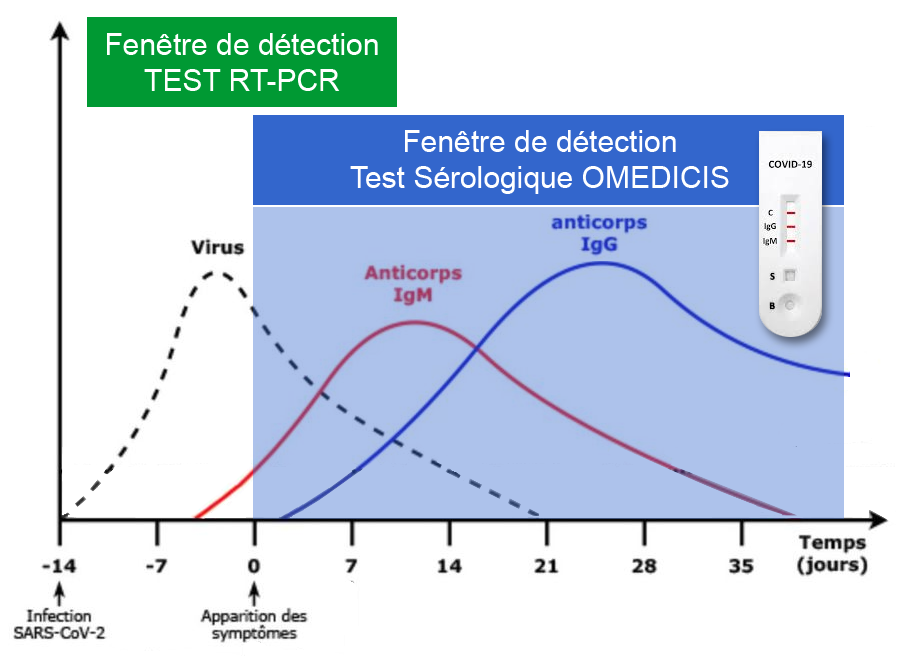
The COVID-19 rapid test is a serological test used to qualitatively detect IgG and IgM antibodies to the novel coronavirus in blood. It can therefore tell whether you are infected or have been infected with the COVID-19 coronavirus.
The rapid tests are based on the principle of lateral flow immunochromatography and are available in cassette form, which is very easy to use. IgM and IgG are antibodies produced by the immune system when a person is infected with the coronavirus, to provide protection against SARS-CoV-2. These anti-SARS-CoV-2 IgM and IgG antibodies can therefore be detected in a person’s blood, and serve as an indicator for detection tests.
Results are visible 10 minutes after the sample and buffer have been combined in the test sample well.
To test the clinical sensitivity and specificity of the new Coronavirus IgM / IgG Antibody Combination Test Kit (2019-nCoV) (Colloidal Gold), blood samples were collected from COVID-19 patients from four hospitals.
The tests were conducted separately at each site. A total of 603 samples were tested: 211 clinically confirmed positive patients and 392 negative patients (not infected with SARS-CoV-2).
For the IgM test, 202 samples tested positive out of 211 confirmed samples, resulting in a sensitivity of 95.73%. 3 samples tested positive out of 392 negative samples, generating a specificity of 99.23%.
For the IgG test, 33 confirmed samples were taken because the samples were taken too early for the patients to generate IgG antibodies. Thus, 3 samples tested negative out of 178 confirmed samples, resulting in a sensitivity of 98.31%. 2 samples tested positive out of 392 negative samples, generating a specificity of 99.23%.
If you have any doubts about the result, you can perform a second test at an interval of 7 days.
The kit includes all the elements to perform this manipulation. Unlike traditional blood tests, the finger prick is very quick, painless (because the needle is very small) and easy to perform.
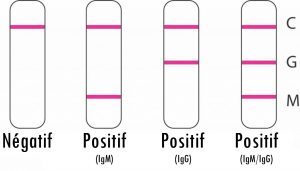
The test includes three detection lines:
The control line (C) appears when the blood sample has passed through the cassette (this line simply tells you that you have performed the manipulation correctly).
- 1 | Negative result*: If only the quality control line (C) appears and the G and M detection lines are not visible, no new coronavirus antibodies were detected and the result is negative.
- 2 | Positive result, M only: If the quality control line (C) and the detection line M appear, then the new IgM antibody to the coronavirus has been detected and the result is positive for IgM antibody.
- 3 | Positive result, G only: If the quality control line (C) and the detection line G appear, then the new coronavirus IgG antibody has been detected and the result is positive for IgG antibody.
- 4 | Positive result, G and M: If the quality control line (C) and both the G and M detection lines appear, then the new IgG and IgM coronavirus antibodies have been detected and the result is positive for IgG and IgM antibodies.
Negative results do not prevent infection with COVID-19 and should not be used as the sole basis for patient management decisions. Negative results should be combined with clinical findings, patient history and epidemiological information.
The test is available for sale on this site in boxes of 1, 5 and 20 tests. These tests are products for laboratories and health professionals.
Once you have placed your order, you will be redirected to an order confirmation message, which will contain your order number. This information will also be sent to you by email. If you do not receive it, please check your spam folder to make sure your confirmation email was not sent there.
Once our warehouse team has processed your order, you will receive a second email to inform you that your order has been shipped. Again, if an email does not seem to have been received, please check your spam folder.
We are sorry to hear that you have received a damaged product. Please send us a message with your order number, a picture of the defect and other details of the problem, within 7 days of receiving your order.
You can contact our customer service and support team via the email box info@omedicis.com. Our customer service team will work diligently to resolve this issue as quickly as possible.
We are very quick in processing orders to ensure that you receive them as quickly as possible.
Once the order has been confirmed, we cannot cancel your order, as our warehouse team will have already started processing and preparing your order for delivery.
Once your order is received and you still wish to cancel your order, simply contact us at info@omedicis.com to notify us that you wish to cancel your order and return it. Once we receive your package, we will contact you to notify you that we have issued a refund for your products.
See this page for more information: Refund Policy.
To contact us, you can use the address info@omedicis.com or our contact form. We will get back to you shortly.
Protections against Covid-19 :
Is it necessary to undergo a serological test before the first dose of vaccine?
It would seem that the HAS is in favour of a general trivialization of the identification of people who already have antibodies against Covid-19 among the volunteers when they take the vaccine.
This provision is all the more likely as its president, the HAS, is working on a document that will institute a generalization of serological tests at the time of the injection of the first dose of vaccine against Covid-19. This is to identify people who may have already developed antibodies without knowing it.
By the way, as of March 1, 2021, a diagnosed Covid-19 infection, is considered a first dose as it is considered a “weak immune response”. Especially since very few recontaminations have been detected in the world compared to the tens of millions of recorded cases.
This leads to the injection of the single dose at least three months after the end of the symptoms. This dose therefore acts as a reminder, to reinforce the protection already in place. It would appear that this practice results in a better immune response than the usual two doses injected into the “uninfected”, according to some scientific studies.
Therefore, one dose is sufficient for those who have had Covid-19, regardless of the time between the two events. Except that many volunteers arrive at the vaccination centre not knowing that they have already contracted an asymptomatic form of Covid-19 and that a single dose would be sufficient.
Vaccine: a serological test is recommended to know if you only need one dose
The French National Authority for Health has just recommended that a serological test be carried out before vaccination to determine previous contamination with Covid-19.
Because if you have already contracted Covid-19, you will only need one shot of the vaccine instead of two. This will be determined by the serological tests deployed in the vaccination centres.
What is a serological test?
This is a blood test that can determine in a few minutes the presence of antibodies developed as a result of infection with Covid-19, regardless of when the infection occurred. These appear within days or weeks of contracting the virus.